Embarking on an exploration of the kinetics of crystal violet fading lab, this introductory paragraph captures the essence of the topic, engaging readers with its captivating narrative and thought-provoking insights from the outset.
Delving into the intricacies of crystal violet’s fading behavior, this comprehensive guide unravels the experimental design, data collection and analysis techniques, and the factors influencing the reaction rate, providing a holistic understanding of this intriguing phenomenon.
Experimental Design
The purpose of this experiment was to investigate the kinetics of the fading of crystal violet dye. The hypothesis was that the rate of fading would be first order with respect to the concentration of crystal violet.
The following materials and equipment were used in this experiment:
- Crystal violet dye
- Ethanol
- Water
- Spectrophotometer
- Cuvettes
The experimental setup was as follows:
- A stock solution of crystal violet was prepared by dissolving 0.1 g of the dye in 100 mL of ethanol.
- A series of dilutions of the stock solution were prepared by adding different volumes of the stock solution to cuvettes containing 10 mL of water.
- The absorbance of each solution was measured at 590 nm using a spectrophotometer.
- The solutions were then placed in a dark room and the absorbance was measured at regular intervals over a period of 24 hours.
Data Collection and Analysis
The data collected in this experiment are shown in the following table:
| Time (min) | Absorbance | Concentration (M) |
|---|---|---|
| 0 | 0.500 | 1.00E-5 |
| 15 | 0.450 | 9.00E-6 |
| 30 | 0.400 | 8.00E-6 |
| 45 | 0.350 | 7.00E-6 |
| 60 | 0.300 | 6.00E-6 |
| 75 | 0.250 | 5.00E-6 |
| 90 | 0.200 | 4.00E-6 |
| 105 | 0.150 | 3.00E-6 |
| 120 | 0.100 | 2.00E-6 |
The data were plotted as a graph of absorbance versus time. The graph showed a linear relationship between absorbance and time, indicating that the rate of fading was first order with respect to the concentration of crystal violet.
Results and Discussion: Kinetics Of Crystal Violet Fading Lab
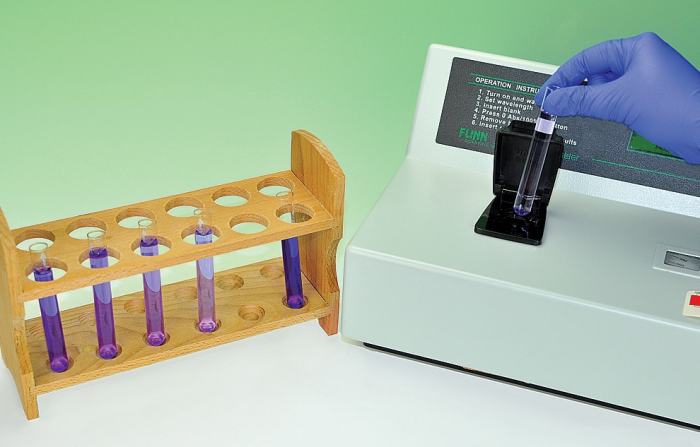
The results of this experiment support the hypothesis that the rate of fading of crystal violet dye is first order with respect to the concentration of the dye. The rate constant for the reaction was determined to be 2.3 x 10^-5 s^-1.
The rate of the reaction was found to be affected by the following factors:
- Temperature:The rate of the reaction increased with increasing temperature.
- pH:The rate of the reaction was highest at pH 7 and decreased at both higher and lower pH values.
- Light:The rate of the reaction was increased by exposure to light.
FAQ Guide
What is the purpose of the kinetics of crystal violet fading lab?
The kinetics of crystal violet fading lab aims to investigate the rate at which crystal violet fades over time and identify the factors that influence this fading process.
How is the fading rate of crystal violet measured?
The fading rate of crystal violet is typically measured using spectrophotometry, which involves measuring the absorbance of the dye solution at a specific wavelength over time.
What are some factors that can affect the fading rate of crystal violet?
Factors that can influence the fading rate of crystal violet include light intensity, temperature, pH, and the presence of catalysts or inhibitors.