A data safety monitoring board report for an investigator initiated – A data safety monitoring board report for an investigator-initiated trial is a critical component of ensuring the safety and integrity of clinical research. This report provides a comprehensive overview of the data safety monitoring board’s (DSMB) role, responsibilities, and reporting requirements, offering valuable guidance for investigators, sponsors, and researchers involved in investigator-initiated trials.
The DSMB plays a crucial role in safeguarding the well-being of research participants by reviewing and monitoring safety and efficacy data throughout the trial. This report delves into the composition and qualifications of a DSMB, emphasizing the importance of independence and objectivity in its decision-making.
Data Safety Monitoring Board (DSMB) Composition and Responsibilities: A Data Safety Monitoring Board Report For An Investigator Initiated
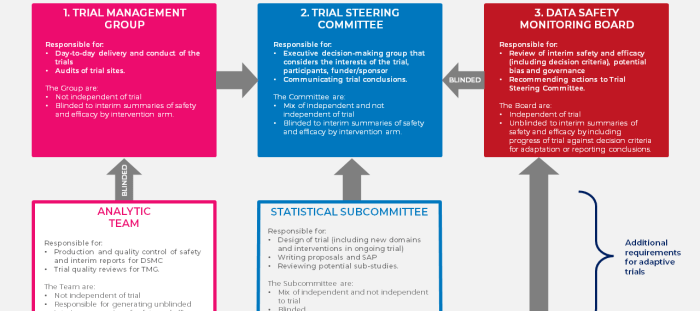
A DSMB is an independent body that oversees the safety and efficacy of clinical trials. In investigator-initiated trials, the DSMB is responsible for:
- Reviewing and monitoring safety data
- Assessing the efficacy of the intervention
- Making recommendations to the investigators and sponsor regarding the continuation, modification, or termination of the trial
The DSMB is typically composed of:
- Statisticians
- Clinicians
- Bioethicists
DSMB members should be independent of the investigators and sponsor and have no financial or other conflicts of interest.
Data Review and Monitoring Process
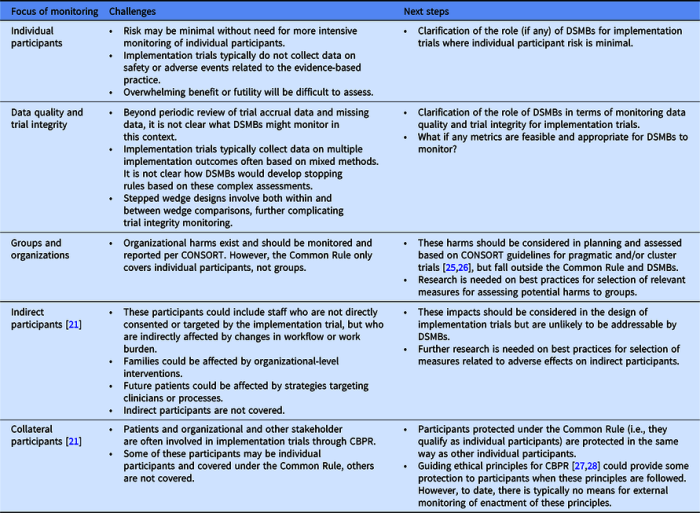
The DSMB reviews data from the trial on a regular basis, typically every 6-12 months. The data is reviewed for:
- Safety concerns
- Efficacy signals
- Compliance with the trial protocol
The DSMB uses a variety of criteria to assess the safety and efficacy of the intervention, including:
- The number and severity of adverse events
- The change in the primary outcome measure
- The compliance with the trial protocol
Based on its review of the data, the DSMB may recommend that the trial be continued, modified, or terminated.
DSMB Reporting
| Element | Content |
|---|---|
| Introduction | A brief overview of the trial and the DSMB’s role |
| Data Review | A summary of the data reviewed by the DSMB, including the number and severity of adverse events, the change in the primary outcome measure, and the compliance with the trial protocol |
| Safety Assessment | The DSMB’s assessment of the safety of the intervention, including any concerns or recommendations |
| Efficacy Assessment | The DSMB’s assessment of the efficacy of the intervention, including any signals of benefit or harm |
| Recommendations | The DSMB’s recommendations to the investigators and sponsor regarding the continuation, modification, or termination of the trial |
DSMB reports should be timely, transparent, and accessible to all stakeholders.
DSMB Interactions with Investigators and Sponsors
The DSMB communicates with the investigators and sponsor on a regular basis, typically through written reports and teleconferences. The DSMB may also meet with the investigators and sponsor in person to discuss the trial and its findings.
The DSMB plays an important role in addressing concerns raised by investigators or sponsors. The DSMB may also recommend modifications to the trial design or conduct to address these concerns.
If there is a disagreement between the DSMB and the investigators or sponsor, the DSMB may refer the matter to an independent arbitrator for resolution.
FAQ Summary
What is the role of a DSMB in investigator-initiated trials?
A DSMB is an independent group of experts responsible for reviewing and monitoring safety and efficacy data in investigator-initiated trials to ensure the well-being of research participants.
What are the key elements of a DSMB report?
A DSMB report typically includes information on the DSMB’s composition, qualifications, data review process, safety and efficacy assessments, recommendations, and communication channels with investigators and sponsors.
Why is DSMB independence important?
DSMB independence is essential to ensure that the board’s decisions are free from bias or undue influence from investigators or sponsors, safeguarding the objectivity and integrity of the safety monitoring process.